Because of its unique properties, lithium and its compounds are widely used in chemical engineering, energy, medicine, metallurgy, etc., and are known as the strategic elements of the 21st century. Lithium resources mainly exist in solid mines and lithium-containing liquid resources (salt lake brine, sea water, geothermal water, etc.). With the decreasing amount of solid resources, the importance of comprehensive development and utilization of brine lithium resources has become increasingly prominent. At present, the main methods for extracting lithium from liquid lithium resources are solvent extraction, membrane, adsorption, etc. Different methods are suitable for specific salt lake brines. Among them, the adsorption method is suitable for the separation of low-grade lithium resources from salt lake brines with high magnesium-to-lithium ratio and potassium extraction tailings. Spinel-type lithium manganese oxide has a unique pore structure, large specific surface area, and high selectivity to lithium. It has a good application prospect as an adsorbent for extracting lithium from a solution.
At present, the most studied lithium manganese oxide compounds are LiMn2O4, Li4Mn5O12 and Li1.6Mn1.6O4. Compared with the former two, Li1.6Mn1.6O4 has the highest theoretical adsorption capacity, but its dissolution loss rate is still far from the actual application. Element doping is an effective method for manganese oxide compounds to improve stability and reduce the rate of dissolution loss. Therefore, how to effectively reduce the dissolution rate of materials while ensuring the adsorption capacity has attracted the interest of many researchers. The current doping work mainly focuses on the replacement of Mn at the 16d site in the Li1.6Mn1.6O4 crystal lattice by transition metals, and there is very little doping of Li1.6Mn1.6O4 crystals with alkali metals and alkaline earth metals. Since the actual lithium ion exchange capacity is only about 60% of the theoretical exchange capacity, the reason may be that part of the lithium acts as a supporting framework. In order to reduce its resistance to dissolution, doping and substitution of lithium ion sites that are difficult to exchange, and the influence of doping and substitution on the structural stability need to be investigated experimentally and theoretically.
At present, there has been a lack of research on the cation skeleton spinel structure composed of alkali metals and transition metals, the doping sites of alkaline earth metal ions and the effect on performance. ISL inorganic separation and inorganic materials group synthesized Mg2+ doped spinel Li1.6Mn1.6O4 (LMO) by hydrothermal method. After demolding, Mg2+ doped spinel lithium ion sieve H1.6Mn1.6O4 (HMO) was obtained. The adsorption results of Li+ show that the adsorption capacity of HMO is similar to that of the matrix ion sieve, and Mg2+ doped HMO has better recycling performance. Compared with the undoped r-LMO after Mg2+ doping, the Mn dissolution loss is reduced by 18.5%. Theoretical studies have shown that Mg2+ replaces Li+ at the 16d site of the bulk phase. The Mg-O bond is stronger than the Li-O bond, which enhances the structural stability of the material; at the same time, after Mg2+ doping, it reduces the charge density near the 16d site. The average valence of Mn is improved, and the dissolution loss of Mn is reduced. This study provides an idea for improving the dissolution resistance of lithium ion sieves: different sites have different properties of lithium. In addition to the 16d site Li+, the higher density 8a site lithium can also be structurally modified to adjust the local charge distribution to achieve. The purpose of stabilizing the framework structure and improving the resistance to dissolution. For details, please see "Research Highlights": 1-14, No. 2 Chemistry Special Issue of "Salt Lake Research" in 2020.
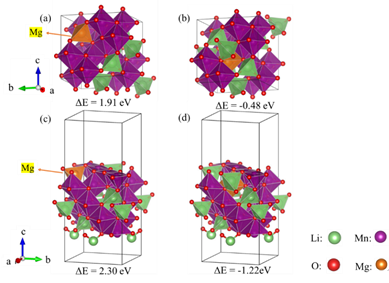
Optimized structure of replacing different Li sites with Mg
