The Solution Structure and Interface Research Group at the Chinese Academy of Sciences Qinghai Institute of Salt Lakes, in collaboration with Jiangsu University and Tianjin University, has conducted a series of innovative studies focusing on the efficient extraction and separation of boron from salt lake brine. With the support of the Qinghai Institute of Salt Lakes Basic Research Young Innovation Cross-Disciplinary Team Program (ISLJCTD-2022-04) and the National Natural Science Foundation of China Regional Development Joint Fund Project (U22A20413), they successfully developed various adsorption separation materials for salt lake boron resources with high adsorption capacity, excellent selectivity, and good stability, providing potential new materials and technological pathways for the efficient separation of boron resources from salt lakes.
Aiming at the problems of prone stacking disorder and limited accessibility of functional sites in Covalent Organic Frameworks (COFs) adsorbents during practical synthesis, a structural reconstruction strategy based on side-chain engineering was proposed. Functionalization was achieved by anchoring polyol side chains onto the units, while simultaneously enhancing the stability of the units, which strengthened the crystalline order of the COFs. After stabilization modification and template removal, a hollow-structured COF adsorbent (HSPCOF) with a large specific surface area and polyol functionalization was obtained successfully. This material combines a high specific surface area, connected pore channels, and excellent chemical stability, achieving a boron adsorption capacity of 150.05 mg g⁻¹ at 298 K, which is more than 10 times that of the commercial resin MK51. Density Functional Theory (DFT) calculations indicated that the polyol side chains can form bidentate cyclic ester complexes with borate anions, thereby endowing the material with high selectivity and strong binding capability. HSPCOF exhibited an excellent stability and a boron removal rate of 91.51% even in the actual salt lake brine,, confirming its application potential in complex systems. This research not only elucidated the growth and reconstruction mechanism of COFs on silicon template surfaces but also proposed a universal strategy for achieving structural ordering and precise functional regulation through side-chain engineering, providing new ideas for the controllable preparation and efficient separation application of hollow functionalized COF-based boron adsorption materials. This work was published in the internationally renowned journal Angewandte Chemie International Edition (IF=16.9, Q1 TOP), with Wenqing Wang (a PhD student jointly trained by Jiangsu University and Qinghai Institute of Salt Lakes,CAS) is the first author.
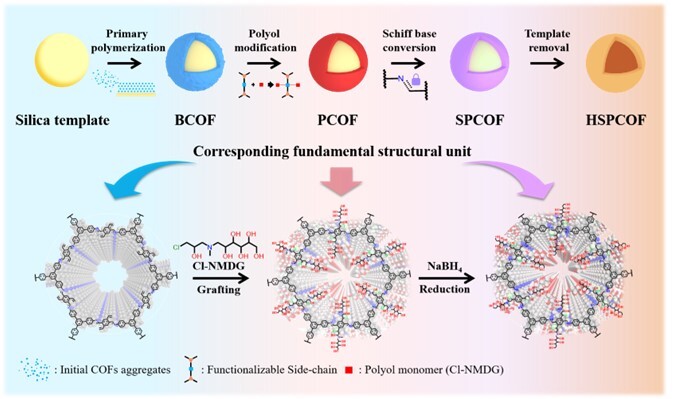
Figure 1. Schematic diagram of the HSPCOF synthesis route. The upper part shows the full synthesis process of HSPCOF; the lower part shows the component structures corresponding to the key steps BCOF, PCOF, and SPCOF.
Another work focused on metal-organic framework-derived hollow cobalt sulfide materials. Using ZIF-67 as a precursor, the team successfully prepared a Co₃S₄ nano-adsorbent with a hollow structure through a solvothermal sulfidation reaction. The adsorption capacity of this material reached 283.3 mg·g⁻¹ in a solution with a boron concentration of 200 mg·L⁻¹, which is 7.3 times higher than that of ZIF-67, and the adsorption equilibrium could be reached within 30 minutes. Kinetic and thermodynamic studies showed that the adsorption process conforms to the Langmuir model, indicating a spontaneous monolayer chemisorption. Through XPS, FT-IR, and DFT calculations, the research further confirmed that the cobalt atoms on the material's surface are the main adsorption sites, the primary driving force for chemisorption is chemical bonding. Electrostatic interactions and hydrogen bonding synergistically achieve the efficient boron capture. It also demonstrated good selective adsorption capability in actual salt lake brines (from Cha'erhan and Laguo Cuo). This work was published in the well-known separation science journal Desalination (IF=9.8, Q1 TOP), with Shao Yifan, Assistant Researcher at Qinghai Institute of Salt Lakes, as the first author.
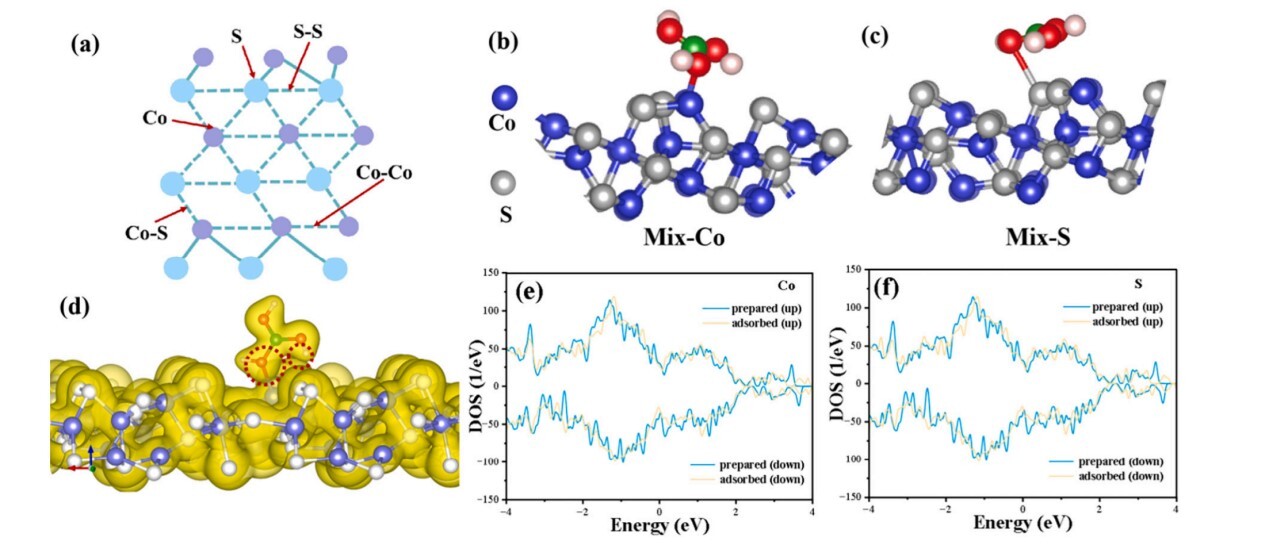
Figure 2. (a) Schematic diagram of adsorption sites on the ZIF-67-derived Co3S4 hollow adsorbent; (b-c) The most stable structure of H3BO3 adsorbed on Co₃S₄ (Co and S sites); (e, f) DOS diagrams of H3BO3 adsorbed on Co₃S₄ at different sites.
The aforementioned two works systematically explored the mechanism of hollow structure design and surface functionalization in enhancing boron adsorption performance, starting from two types of the novel materials: Covalent Organic Frameworks and Metal-Organic Framework-derived materials, respectively. They not only provide new material systems for solving the challenge of boron separation in high-salinity brine but also offer theoretical basis and technical references for designing functional adsorbents for the efficient capture of specific ions in complex water bodies.
Paper Information:
[1] W. Wang, H. Li, Y. Huang, Y. Zhou, J. Pan, Z. Jiang. Side-Chain Engineering of Hollow Spheres of Covalent Organic Frameworks for High-Efficiency Boron Capture from Brine. Angew. Chem. Int. Ed. 2025, e20582.
https://doi.org/10.1002/anie.202520582
[2] Y. Shao, C. Hu, R. Liu, B. Liu, Y. Zhou, J. Pan, Selective and Efficient Adsorption of H₃BO₃ from Salt Lake Brine Solution by ZIF-67-Derived Hollow Cobalt Sulfide. Desalination 2025, 613, 119019.
https://doi.org/10.1016/j.desal.2025.119019
