Recently, the Ion Adsorption Science and Technology Research team at Qinghai Institute of Salt Lakes, CAS has achieved new progress in electrochemical ion extraction. To address the challenges of low capacity, poor selectivity, and slow kinetics in lithium extraction from brine with high Na/K/Mg ratios, the team pioneered the application of electroactive organic molecular ion-adsorbent materials for brine resource extraction.
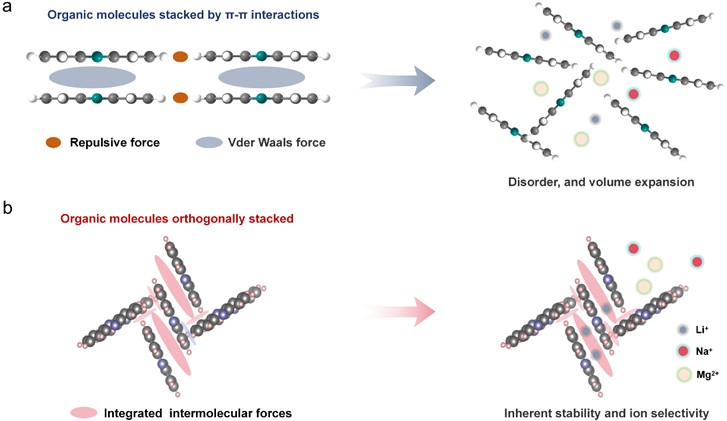
To overcome structural collapse caused by ion-intercalation-induced volume expansion, the team proposed an innovative strategy for stabilizing crystal structures through intermolecular force networks. Using phenazine as a model system, orthogonal molecular stacking was proven to create robust intermolecular networks and ion-selective nanochannels. This structure not only mitigates mechanical failure from volume expansion but also provides lithiophilic nanochannels with atomic-scale Li⁺ selectivity, achieving a record capacity of 109 mg/g. This study fundamentally elucidates the physicochemical principles linking molecular stacking to macroscopic electrochemical stability and ion selectivity.
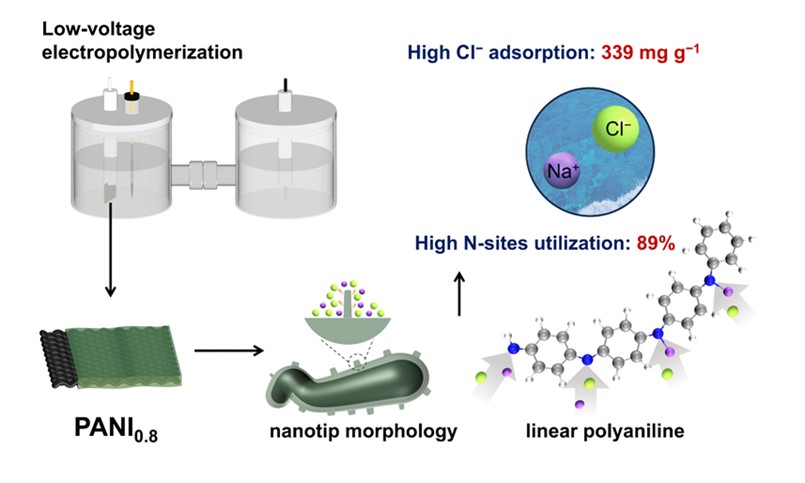
Polyaniline (PANI) is widely used in energy storage, environmental remediation, and ion separation due to its excellent conductivity and reversible doping properties. In-situ electrochemical spectroscopy confirmed that a "low-potential" strategy suppresses coupling side reactions, yielding oligomeric linear PANI with nanotip structures. This unique architecture enhances nitrogen-site accessibility, achieving breakthrough Cl⁻ (339 mg/g) and Na⁺ (142 mg/g) adsorption capacities.
Published in Nano Letters (DOI:10.1021/acs.nanolett.5c03784) and Chemical Engineering Journal (DOI:10.1016/j.cej.2025.168274)
