A research team led by Prof. Li Wu and Prof. Zhang Bo from the Solution Chemistry Group at the Qinghai Institute of Salt Lakes (QISL) has made a breakthrough in the development of wide-temperature-range magnesium-based lithium-ion batteries. By designing an "alkyl-chain sway" mechanism at the cathode interface, the team successfully unified the performance enhancement mechanisms of lithium-ion batteries at both high and low temperatures, achieving a leap in cycling stability across extreme temperatures compared to previously reported studies.
The wide-temperature performance of lithium-ion batteries directly determines their applicability in harsh environments. After years of exploration, researchers have reached a consensus that the cathode interfacial process is essential for achieving long-term cycling stability across temperature ranges. However, the mechanisms for enhancing battery performance at high and low temperatures differ drastically, making this task highly challenging. Inspired by the movement of animal spermatozoa, which alter surrounding fluid dynamics through flagellar motion, the team introduced an "alkyl-chain sway" design at the cathode interface. This innovation significantly improves electrolyte flow at the interface, enabling rapid formation of a stable solid electrolyte interphase (SEI) film. The design not only protects the cathode material from electrolyte corrosion at high temperatures but also accelerates lithium-ion transport in highly viscous electrolytes at low temperatures, thereby boosting performance under both conditions. In this work, the Tween80 first serves as the framework of the weak-linked flexible confined space for preparing ultrafine nano-Mg(OH)2 and then adsorbs in situ onto the nano-Mg(OH)2 surface. The nano-Mg(OH)2 improves the surface lithiophilicity of cathodic LiNi0.5Co0.2Mn0.3O2, and induces the formation of stable and conductive inorganic interphase, as well as the carrier of Tween80. The unstable coordination of lithium ions to Tween80 cause continuous change of molecular charge state, leading to the alkyl chain sway in the electric field. The half-cell maintains over 70 mAh·g−1 and 90% Coulombic efficiency after 1000 cycles at 60 °C, and keeps 80 mAh·g−1 with 99% Coulombic efficiency after 500 cycles at −5 °C, and remains stable even at −15 °C.
The findings, titled "Lithium-Ion Batteries with Superlong Cycle-Life in Wide Temperature Range via Interfacial Alkyl-Chain Sway," were published in the journal of Advanced Materials (Impact Factor: 27.4). The study was conducted under the leadership of Prof. Li Wu, head of the Solution Chemistry Group, with Prof. Zhang Bo’s team playing a central role. Ph.D. candidate Huang Jinwang from QISL is the first author, while Prof. Ye Xiushen and other QISL researchers provided critical supports. Prof. Zhang Bo and Prof. Ye Xiushen are the co-corresponding authors.
This research benefited from the robust scientific foundation established by earlier generations of scientists at QISL. Funding was provided by the Qinghai Provincial Department of Science and Technology Applied Basic Research Project (2023-ZJ-758) and the "Kunlun Talents·High-End Innovation and Entrepreneurship Talent" Program-Leadership Project.
Article link: https://doi.org/10.1002/adma.202500394
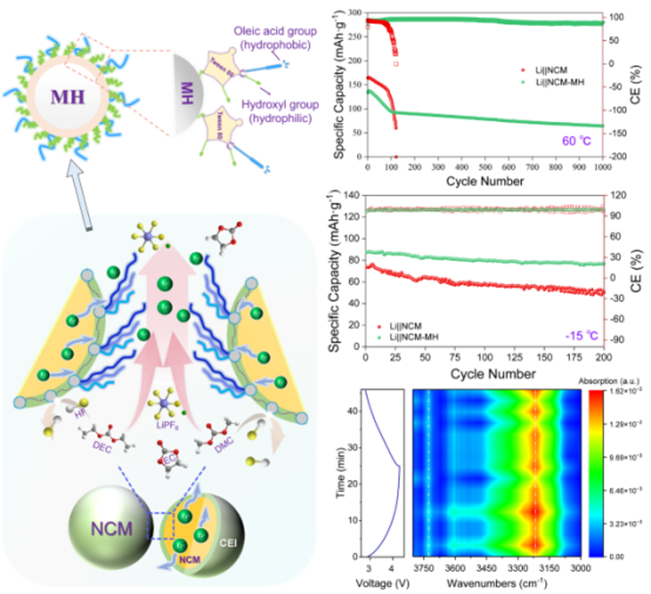
Schematic Diagram of the Alkyl Chain Swing Mechanism and
Comparison of Battery Long-Term Cycling Performance
