Ion exchange membranes (IEMs) are pivotal in extensive technologies, such as lithium extraction from salt lakes, seawater desalination, and fuel cells. Their performance hinges on selective ion transport, governed by the selectivity coefficient SFij, which depends on the thermodynamic partitioning (Ki/Kj) of ions i and j between membrane and external aqueous solutoin and diffusion coefficient (Di/Dj) of ions i and j in membrane , as expressed by eq. (1)
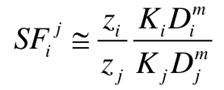 (1)
(1)
Even the Donnan equilibrium theory , i.e., eq. (2) with γ+m, γ−m , γ+s, and γ−s are unity and superscript m, and s indicate IEM and external solution, explains the dependence of membrane-phase ion concentrations on external solutions. However, the neglect of the non-ideality of the mobile ions in the membrane phase leads to inaccuracies in high-salinity environments. More and more experimental studies have emphasized the importance of the ionic activity coefficients in the IEMs.
 (2)
(2)
To model ionic activity coefficients in membranes, the celebrated Manning’s counterion condensation theory —initially developed for polyelectrolyte aqueous solutions—has been extended to IEM systems, but it didn’t work well. The Manning theory sees the polyelectrolyte as a linear polymer chain with uniform charge density, which deviates significantly from the entangled and cross-linked polyelectrolyte in the IEMs. Moreover, the swollen IEMs have an intrinsic concentrated solution characteristic, considering the relatively low swelling and water content (Figure 1).
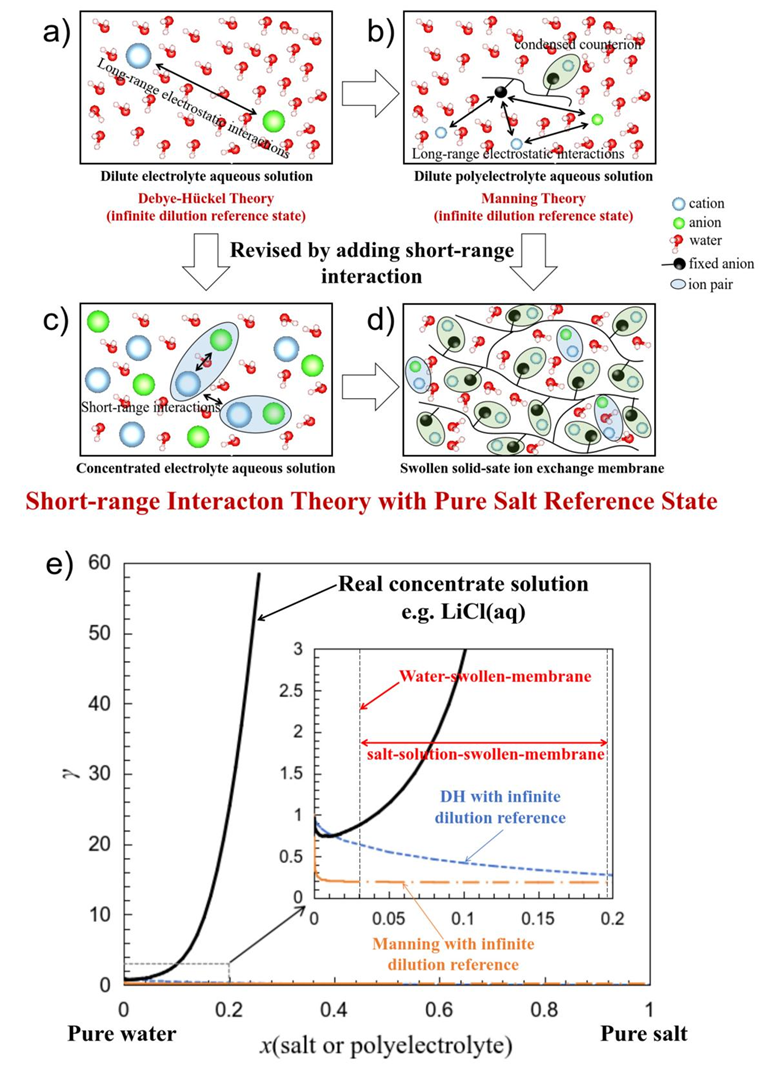
Figure 1. Structural differences among (a) dilute electrolyte, (b) dilute polyelectrolyte, (c) concentrated electrolyte solutions, (d) swollen IEMs, and (e) the typical variation of the ionic activity coefficient in these systems.
Building on insights from concentrated electrolyte solution theory of Kenneth S. Pitzer , the author proposes that the counterion condensation effect in ion exchange membranes (IEMs) is significantly violent than that described by Manning's theory for dilute polyelectrolyte solutions. In water-swollen IEMs, most counterions remain condensed, effectively shielding charges on polymer chains. Consequently, short-range interactions between free ions—rather than long-range electrostatic forces—emerge as the predominant factor governing ion behavior. This perspective aligns perfectly with concentrated electrolyte theories that neglect long-range electrostatic contributions.
Consequently, the author proposed a “Water-Swollen-Membrane Reference State”, which explicitly distinguishes, for the first time, the standard chemical potentials of counterions within the membrane from those in the solution phase. Inspired by the scaling theory of polymer of Pierre-Gilles de Gennes , the author then introduced a “Scaling Regular Solution Theory”, i.e., a power-law relationship between the “effective free counterion concentration” and the “apparent free counterion concentration” in the membrane phase, to describe the excess Gibbs energy the the swollen IEM, i.e., eq. (3).
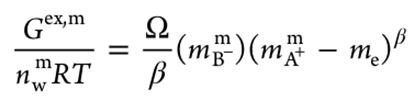 (3)
(3)
This framework achieves a unified description of ion activity coefficients across diverse ion exchange membrane systems, as validated by experimental data spanning the last 70 years (Figure 2). The theory underscores the critical role of the membrane’s intricate polymer network as a cohesive topological entity in governing free ion behavior (left panel of Figure 3). Here, the scaling factor β in eq. (3) serves as a key parameter characterizing the global topological architecture of the membrane polymer, offering novel theoretical guidance for designing advanced ion exchange membranes.
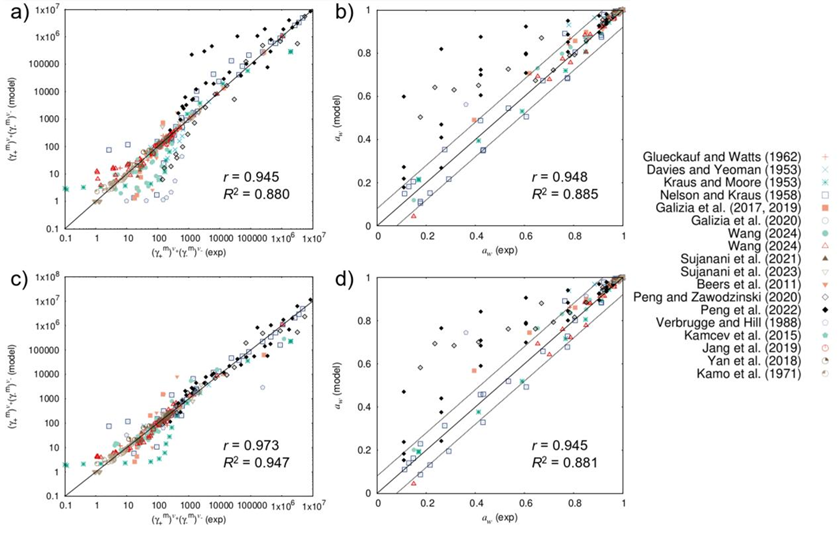
Figure 2. Experimental vs. theoretical ionic activity coefficients, a) and c),
and water activity, b) and d), in IEMs equilibrated with different electrolytes.

Figure 3. Supplementary cover of the Journal of Physical Chemistry B
(2025, 129, 19, 4794-4810.) and the first fage of the article.
This work was supported by the CAS Western Light Program (292022000019), Qinghai Province Kunlun Talents Plan, and CAS Strategic Priority Research Program (XDB1130402).
Article Information: Thermodynamics of Mobile Ion in Ion Exchange Membranes: Water-Swollen-Membrane Reference State and Quasi-Regular Solution Model. J. Phys. Chem. B 2025, 129, 19, 4794–4810. DOI: 10.1021/acs.jpcb.4c08514
