Background
Lithium, a key strategic resource, is widely used in lithium-ion batteries and portable energy storage devices. Salt lakes, one of the world's important sources of lithium, have become a significant source for extracting lithium and addressing the shortage of lithium resources. Among various technologies, the electrochemical adsorption method for lithium extraction has attracted considerable attention due to its advantages, such as a fast rate, large capacity, and environmental friendliness. The design and preparation of electrodes are central to electrochemical adsorption for lithium extraction. This study focuses on the electrochemical adsorption of lithium ions and proposes an innovative electrode: a composite electrode made of poly(vinyl alcohol)-polyaniline copolymer (CP) and H1.6Mn1.6O4 (HMO), aiming to address the mismatch between Li⁺ diffusion and electron transport rates.
Research Content
By coating the ion-electron dual-conducting copolymer (CP) onto the surface of HMO and fabricating it into an electrode, the Li⁺ diffusion coefficient was successfully increased, the solution resistance and charge transport resistance were reduced, and the rate matching between Li⁺ diffusion and electron transport was improved. This synergistic effect not only improves the electrochemical activity of the electrode but also accelerates the Li⁺ adsorption kinetics, enabling an adsorption capacity of up to 49.48 mg/g and demonstrating excellent adsorption performance. This study provides a concept for the development of the next generation of efficient lithium extraction electrodes by simultaneously regulating the diffusion of Li+ and the transport of electrons in the electrochemical adsorption process. The work was published in the journal ACS Nano under the title "Triggering Ion Diffusion and Electron Transport Dual Pathways for High Efficiency Electrochemical Li+ Extraction", with Liu Zhong from the Qinghai Institute of Salt Lakes, Chinese Academy of Sciences, and Chen Hong from Southern University of Science and Technology as the co-corresponding authors. The core point of this work is to promote the Li+ diffusion and electron transport in the H1.6Mn1.6O4 (HMO) electrode matrix by rationally designing and integrating an ion and electron dual-conducting poly(vinyl alcohol)-polyaniline (PVA-PANI) copolymer (CP). Under the same initial Li+ concentration conditions, the HMO@CP electrode shows a larger adsorption capacity and a faster adsorption rate. Not only that, with the increase of the initial Li+ concentration, its adsorption efficiency still maintains a significant advantage. Compared with the existing literature reports, the HMO@CP electrode achieves efficient Li+ capture at lower concentrations and shorter times. Theoretical calculations further confirm the increase in the binding energy of -OH with Li⁺ and the denser electron density of PANI within the CP framework, thus confirming the observed acceleration of Li⁺ diffusion and electron transport.
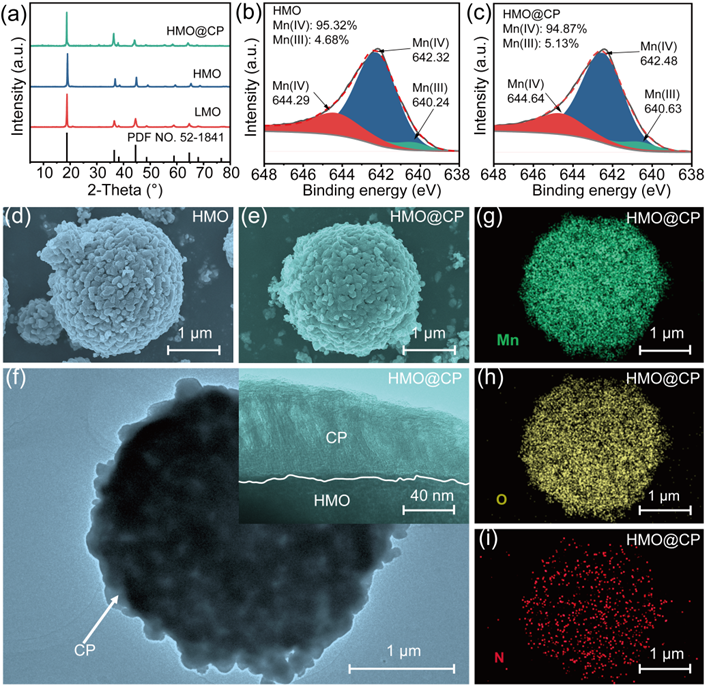
Figure 1. Characterization of the HMO and HMO@CP: (a) XRD patterns; XPS spectra of (b) HMO and (c) HMO@CP; SEM images of (d) HMO and (e) HMO@CP; (f) TEM image of HMO@CP; (g−i) EDS mapping of HMO@CP.
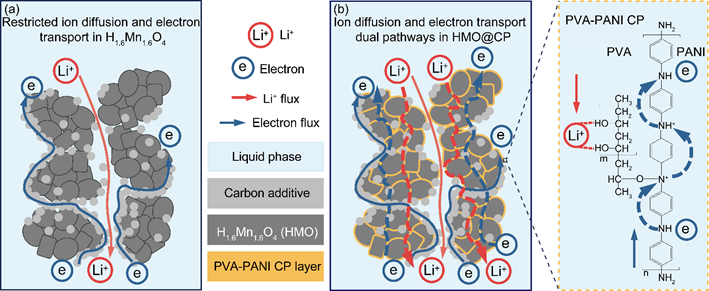
Figure 2. Concept of triggering ion diffusion and electron transport dual pathways for boosted electrochemical Li+ adsorption efficiency: (a) restricted ion diffusion and electron transport in the H1.6Mn1.6O4 electrode; (b) ion diffusion and electron transport dual pathways in the HMO@CP electrode.
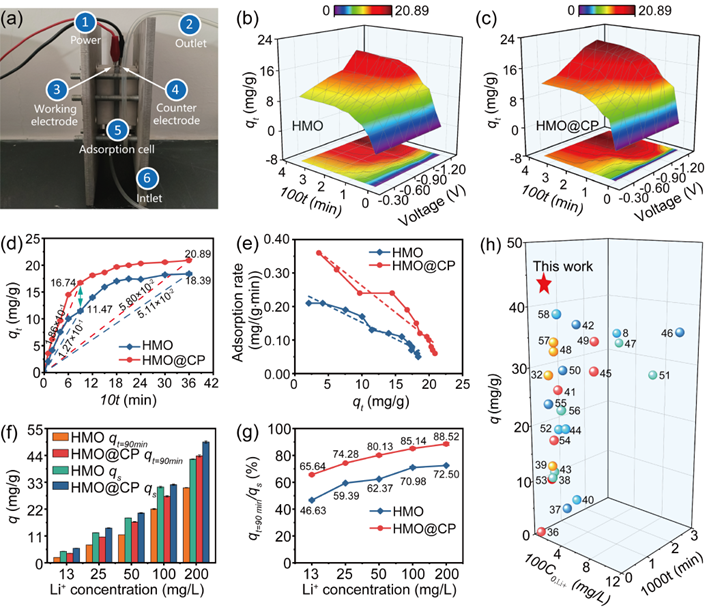
Figure 3. (a) Homemade device used in the continuous electrochemical adsorption experiment; Surface plots of adsorption capacity response to time and applied voltage: (b) HMO electrode and (c) HMO@CP electrode; (d) adsorption curves (E = −1.00 V, C0, Li+ = 50 mg/L); (e) Kim-Yoon plots (E = −1.00 V, C0, Li+ = 50 mg/L); (f) Adsorption capacities at 90 and 360 min under various initial Li+ concentration; (g) qt=90min/qs curves under various initial Li+ concentrations; (h) Comparison of the adsorption capacity in this work with the reported data.
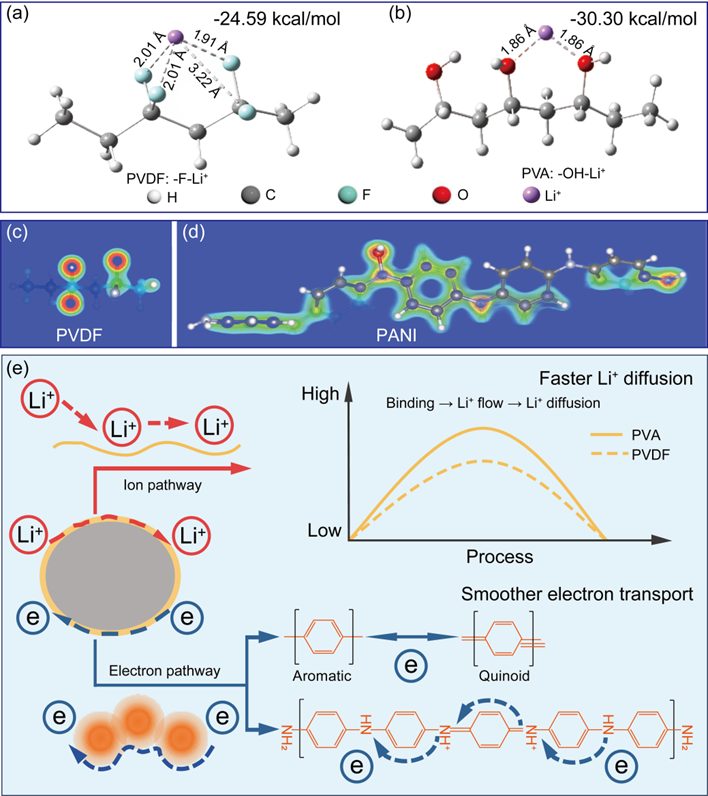
Figure 4. Binding energy: (a) −F-Li+ in PVDF and (b) −OH-Li+ in PVA; electron density distribution: (c) PVDF and (d) PANI; (e) proposed mechanism for triggering ion diffusion and electron transport dual pathways for boosted electrochemical Li+ extraction efficiency.
Author's Biography

Corresponding Author: Liu Zhong, professor, and deputy director of the Laboratory of Salt Lake Resources Chemistry, Qinghai Institute of Salt Lakes, Chinese Academy of Sciences. He is the project leader of the Youth Team Project in the Field of Basic Research Supported by the Chinese Academy of Sciences, selected as the leader of the natural science and engineering discipline in Qinghai Province, a high-end innovative talent in Qinghai Province, a member of the Youth Innovation Promotion Association of the Chinese Academy of Sciences, and a type A talent in the "West Light" program for young scholars. He has presided over 3 projects of the National Natural Science Foundation of China, 6 projects at the provincial and ministerial levels, and won the first prize of the Shanxi Provincial Science and Technology Award (Natural Science) (the second finisher), and the first prize of the "Natural Science Excellent Academic Paper Award of Qinghai Province". His main research work focuses on the basic research and complete set of process development of adsorption and separation of lithium, boron, rubidium, caesium, etc. in salt lakes.
Email: liuzhong@isl.ac.cn

Corresponding Author: Chen Hong, professor, currently the deputy secretary of the Party Committee of the School of Environmental Science and Engineering, Southern University of Science and Technology, and the deputy director of the Shenzhen Key Laboratory of Materials Interface Science and Engineering Applications, and the leader of the research group. He mainly engages in research on "resource recycling and utilization chemistry". In recent years, he has published more than 130 papers in magazines such as Nature Materials, Science Advances, PNAS, and Nature Communications. He has received the Excellence Award of the Young Scientist Award of the Chinese Society for Environmental Sciences, the Gold Award of the Ecological Environment Young Science and Technology Award of the Guangdong Society for Environmental Sciences, and was selected for the national-level youth talent project, the Guangdong Provincial Outstanding Youth Fund, and the Shenzhen Peacock Plan Talent Class B. He also serves as the associate editor of Environmental Chemistry Letters (Q1, IF=15); the editorial board member of Sustainable Horizons, Environmental Function Materials, and Industrial Water Treatment; and the young editorial board member of Chinese Chemical Letters and Chemical Engineering Journal Advances.
Email: chenh3@sustech.edu.cn

Co-first Author: Zhan Honglong, a doctoral student, mainly studies the preparation of manganese-based lithium-ion adsorbents and the optimization of electrochemical adsorption performance.
Email: zhanhl@isl.ac.cn

Co-first Author: Qian Zhiqiang, male, associate professor, master's supervisor, mainly studies the adsorption separation science and technology. He was selected into the "Kunlun Talent High-End Innovative and Entrepreneurial Talent Program" in Qinghai Province, directly recognized as a top-notch talent, the "Specialized Backbone Position" of the Chinese Academy of Sciences (category: foundation and original), the "Western Youth Scholar" of the Chinese Academy of Sciences, and a young editorial board member of "Salt Lake Research".
Email: qianzq@isl.ac.cn
