Salt lakes are China's precious treasure trove of inorganic salt resources. Salt lake brines are rich in elements such as potassium, magnesium, sodium, and chlorine. In addition, The overall reserves of lithium, rubidium, cesium, and iodine are also considerable, and their content in brine is as high as tens or even hundreds of times that in seawater. Among them, salt lake lithium resources account for more than 85% of China ’s total lithium reserves, and the plutonium reserves in Chaerhan area are up to 38,000 tons, which belongs to extra-large deposits. Among them, salt lake lithium resources account for more than 85% of China's total lithium storage. And the storage of plutonium in Qarhan region is as high as 38,000 tons, which belongs to extra-large deposits. The rare elements such as lithium, rubidium, cesium, and iodine in salt lakes have special physical and chemical properties, and their simple substances and their compounds have important uses in chemical industry, defense industry, aerospace, and some special industries. In recent years, due to the development of science and technology, the demand for these rare elements and related compounds has increased. However, due to its few independent deposits and scarce resources, the separation and extraction of rare elements in salt lake brines have attracted much attention.
Aiming at the research on the separation and extraction of rare elements in salt lakes, Professor Liu Haining's team of ISL focused on the research of inorganic adsorption materials, summarized the development of emerging technologies, and pointed out the problems and future research directions of rare element adsorption and separation in salt lakes:
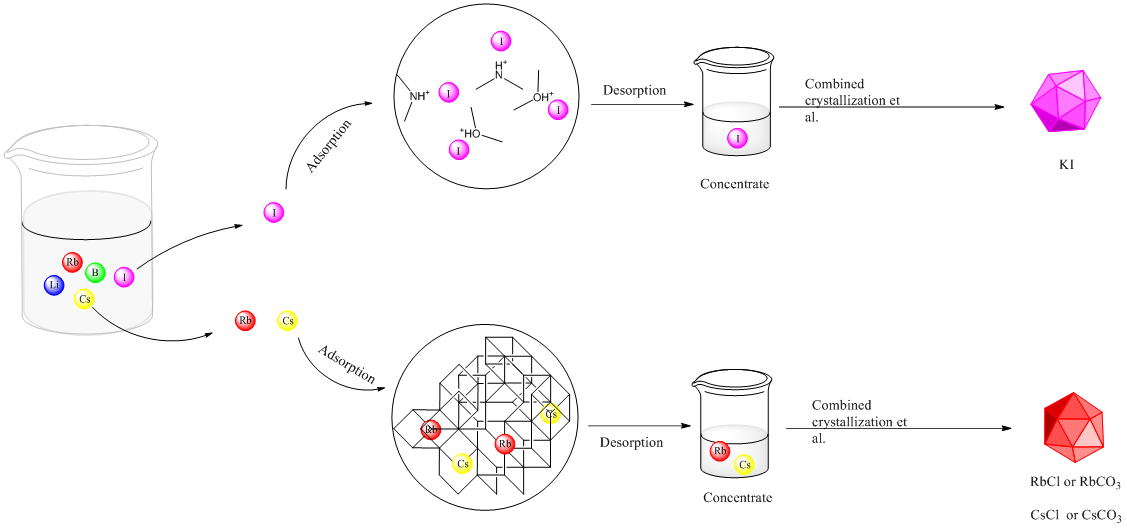
1) From the salt lake system, during the concentration and crystallization of brine, there is a certain degree of loss of rare elements. Therefore, choosing the appropriate stage for adsorption and separation can avoid the loss of rare elements, and can properly avoid the adverse effects of the adsorption process due to changes in brine concentration and other changes in the physicochemical properties of the system;
2) For lithium, rubidium, and cesium, inorganic adsorption materials have obvious adsorption selectivity and capacity advantages, but the molding of materials is still the key to restrict their use;
3) For the separation of iodine, the current research shows that the use of elements such as N and O after protonation for adsorption has better comprehensive performance than inorganic separation materials. The main direction in the future is also to prepare protonated separation materials with stable structure and composition, which is the key to this research;
4) From the perspective of the separation of rare elements in salt lakes, from the perspective of mechanism, competitive adsorption of coexisting ions is inevitable, so it is very difficult to rely on adsorption alone to obtain a single substance with higher purity.
With the continuous emergence of new separation technologies, coupling adsorption with other technologies, such as adsorption-flotation, electrosorption, and special ion selective membranes, is also an effective way to effectively improve the solid-liquid separation of powdered adsorbents. For details, please refer to "Salt Lake Research" Issue 3, 2019 "Salt Lake Chemistry Special Issue" Research Highlights ": pages 11-20.