α-Al2O3 powder has excellent electrical, optical, mechanical, wave absorbing, heat resistance, corrosion resistance and other properties. It is an advanced ceramic raw material that is widely concerned by high-tech fields and academic circles. With the rapid development of science and technology, the preparation method of α-Al2O3 powder is changing with each passing day, and its application performance is inseparable from its preparation method. At present, the purity of α-Al2O3 powder prepared by using the Bayer method with bauxite as the raw material is 99.6-99.9%, which can be applied to refractory materials, spark plugs, IC substrates, etc. While, the α-Al2O3 powder with a purity of> 99% can be used in the fields of high-pressure sodium lamps, transparent tubes for sapphire, high-strength ceramic tools, and tape abrasives. And it also presents increasing application trends in emerging fields such as plasma display materials (PDP powder), lithium-ion battery isolation films, three-primary phosphors for green lighting energy-saving lamps, light-emitting diode substrate materials, and highly active adsorbents.
However, the traditional α-Al2O3 powder cannot meet the application requirements in the above high-end fields due to the large grain size, low comprehensive properties such as strength and toughness, etc. Therefore, the preparation of high-purity α-Al2O3 powder with excellent performance has very important social and economic values.
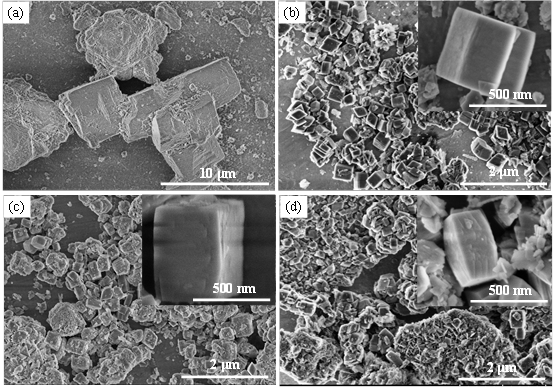
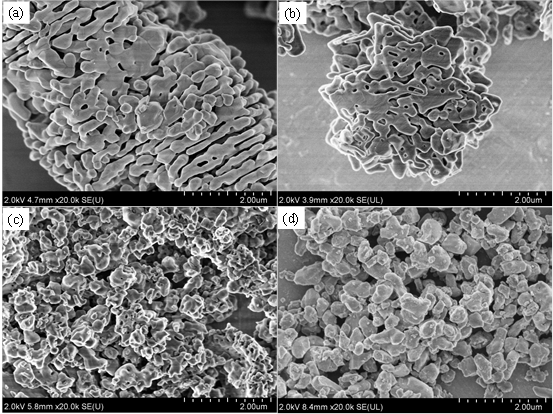
Although the α-Al2O3 powder prepared by the direct hydrolysis method between aluminum powder and water has high purity (more than 3N level), low energy consumption, no environmental pollution and other problems, it has high industrial application prospects. However, how to achieve a controlled hydrolysis reaction and prepare α-Al2O3 nano-powders with controlled morphology and crystal structure is still the technical bottleneck that this preparation method needs to break through. In response to this problem, based on years of research experience, the team of Professor Hai Chunxi of ISL solved the problem of intense hydrolysis and poor controllability through co-solvent screening, and successfully prepared hexagonal star-shaped γ- Al (OH)3 precursor. Then, a high-purity, single crystal structure γ-AlOOH precursor was obtained by hydrolyzing the product directly by hydrothermal treatment. The reaction system of this method is simple, clean and environmentally friendly. According to the purity, structure, morphology, and dispersion characteristics of different types of aluminum hydroxide precursors, the changes and formation mechanisms of the structure and morphology with roasting conditions are systematically studied. These technologies have laid a good technical foundation for the successful preparation of high-quality aluminum hydroxide precursors (γ-Al (OH)3, γ-AlOOH) and α-Al2O3 powders with low energy consumption and high yield. For details, please refer to "Salt Lake Research", Issue 3, 2019, "Salt Lake Chemistry Special Issue" Research Highlights ": pages 1-10.