China is a big energy-consuming country. In recent years, due to the excessive exploitation and use of fossil energy, environmental and ecological problems have become increasingly prominent, seriously affecting and restricting the sustainable development of China's economy. Therefore, research and development of low-emission emissions, stable new energy and high-efficiency energy-saving technologies have become the key to China's energy sector technology. As an important energy-saving technology, energy storage technology has attracted wide attention in various industries in recent years. The core of the energy storage technology is phase-change energy storage materials with low cost and stable performance.
As a solid-liquid phase energy storage material, hydrated salt phase change energy storage material has the advantages of abundant raw materials, high heat storage density, environmental protection, low price, etc.. It has broad market prospects and economic benefits in the efficient use of solar energy, the utilization of industrial waste heat, cross-season heat storage, smart greenhouses, food storage and preservation, and textile industry. However, hydrated salt phase change energy storage materials will encounter excessive cooling, phase separation, non-coordinating melting, low heat transfer efficiency (low thermal conductivity), volume change, cycle stability, slow crystallization rate and heat in practical applications.
In response to the above problems, Professor Zhouyuan Research Team, from Salt Lake Resources Chemistry Laboratory, has improved the performance of hydrated salt phase change energy storage materials and broadened its application range through comprehensive research. This research idea is of great significance for the development of hydrated salt phase change energy storage materials. For details, see Salt Lake Research, No. 2, 2018, “Research Highlights”, pages 9-15.
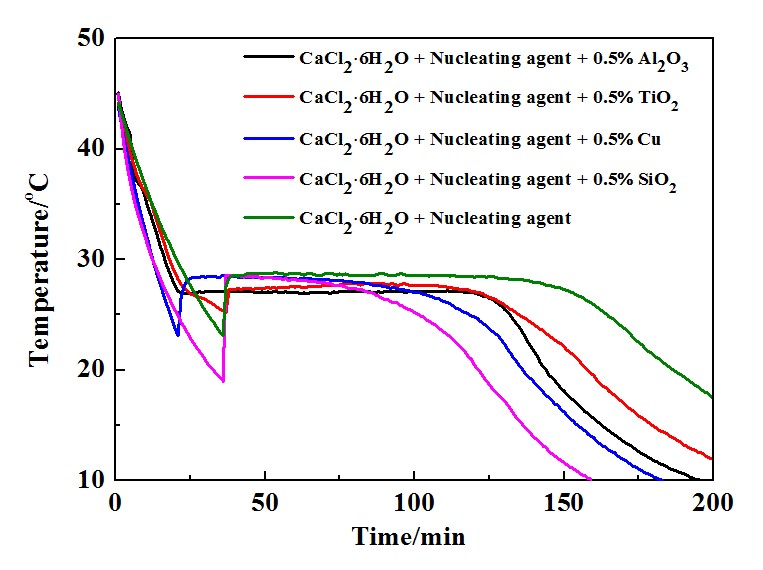
Effect of Nanoparticles on Subcooling of CaCl2·6H2O Phase Change Materials

The enthalpy change of calcium chloride hexahydrate, composite and coated composites in the cycle (a) phase change enthalpy (b) phase transition temperature
