Boron is an element necessary for the origin of life and an important industrial raw material. Borates are not only used in the fields of special glass, ceramic glaze, wood preservation, high-quality flame retardants, peroxyborate bleaching detergents, etc., but also widely used in laser materials, light-emitting matrix materials and nonlinear optical crystal materials.
For a long time, it has been generally believed that many factors affect the species of borate solution, including total boron concentration, pH, temperature, cations and anions, and even the life of the solution. These are all described at a macro level. There are few reports on the structure of the borate solution at the micro level and its combination with the macro level. The main reason is that there are many confusing scientific problems, such as: how do the solid-liquid composition and solid-liquid heterogeneous compounds affect the borate species in solution; how the structure of solvent water affects the structure of borate species; how the various borate species commonly found in solution hydrate; etc.
In response to the above-mentioned scientific problems, in the past ten years, the team of Professor Fang Chunhui of ISL has studied the structure of aqueous solutions of alkali metals and magnesium borate using by contemporary advanced synchronous radiation X-ray scattering, neutron scattering, EXAFS, NMR, Raman, DFT, and MD / CPMD, etc. The solvent, solute and solvated structure of the solution were obtained by scattering method. High atomic number cation hydrated structure obtained from EXAFS, qualitative identification of boron species by Raman and NMR, species distribution in solution was obtained from pH measurement and chemical equilibrium calculations, a detailed picture of the hydration structure around the six-membered ring and a stepwise hydrolysis mechanism were obtained by DFT calculation and MD / CPMD simulation, and was compared with the scattering method and Raman. These results are of great significance to the formation and evolution of borate, the development and utilization of resources, and the recovery and reuse of boron materials. For details, please refer to “Salt Lake Research”, Issue 2 of the 36th International Solution Chemistry Conference, “Research Highlights”: 11-39, 2019.
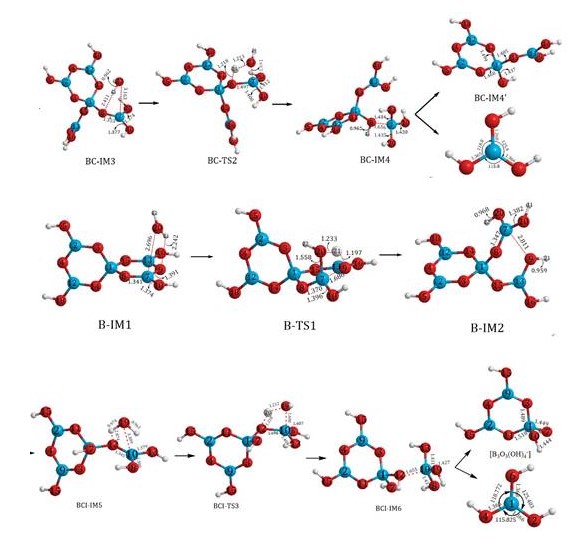
Fig. 1. Schematic diagram of the stepwise hydrolysis pathway of [B5O6 (OH) 4-]