In recent years, the use of lithium and its compounds has become increasingly important and widespread, especially in emerging technologies such as new energy, new materials and information industries. The resources of lithium in nature can be divided into two categories: hard rock, such as spodumene (LiAlSi2O6), lithium mica (K(Li,Al)3(Si,Al)4O10(F,OH)2), lithium long Stone (LiAlSi4O10); and brine, such as salt lake brine, oil field water, underground brine, and the like. Chile's SQM, the world's largest lithium salt production company, produces a variety of lithium compounds from the famous Atacama salt lake brine in the Central Andes.
China's Qinghai-Tibet Plateau is one of the most concentrated areas of the world's rich lithium salt lakes. The carbonate type, magnesium sulfate subtype, sodium sulfate subtype and chloride type salt lake are famous for their high content of lithium and boron. However, for the separation and extraction of lithium salts from salt lake brines, the concentration of lithium is too low. Even if Atacama, the highest concentration of lithium in the world salt lake brine, is from the salt lake, the lithium in the brine is only 0.15%. Therefore, in order to extract lithium from salt lake brine, it is necessary to evaporate and enrich the brine, which is inseparable from the use of salt field process technology.
For the salt-salting sequence during the evaporation of lithium salt, especially the salt-forming behavior of lithium, the team of Professor Song Pengsheng of ISL proposed the six-element system Li, Na, K, Mg/Cl, SO42--H2O as early as 2003. 25 ° C thermodynamic model. And it is indicated that the thermodynamic model is the only tool for predicting the equilibrium conditions in the evaporation process of Li+, Na+, K+, Mg2+/Cl-, SO42--H2O solutions. In recent years, the team used the above thermodynamic model to conduct evaporation experiments on a number of salt lake brines, and calculated the crystal precipitation sequence of 25 kinds of lithium-rich brines during 25 °C isothermal evaporation, and discussed the precipitation and formation of lithium salts. These laws not only enrich our knowledge of lithium salt chemistry, but also have important guiding significance for the salt field process of lithium brine lake brine processing. For details, please refer to Salt Lake Research, No. 2, 2019, the 36th International Conference on Solution Chemistry, "Research Highlights": 1-10.
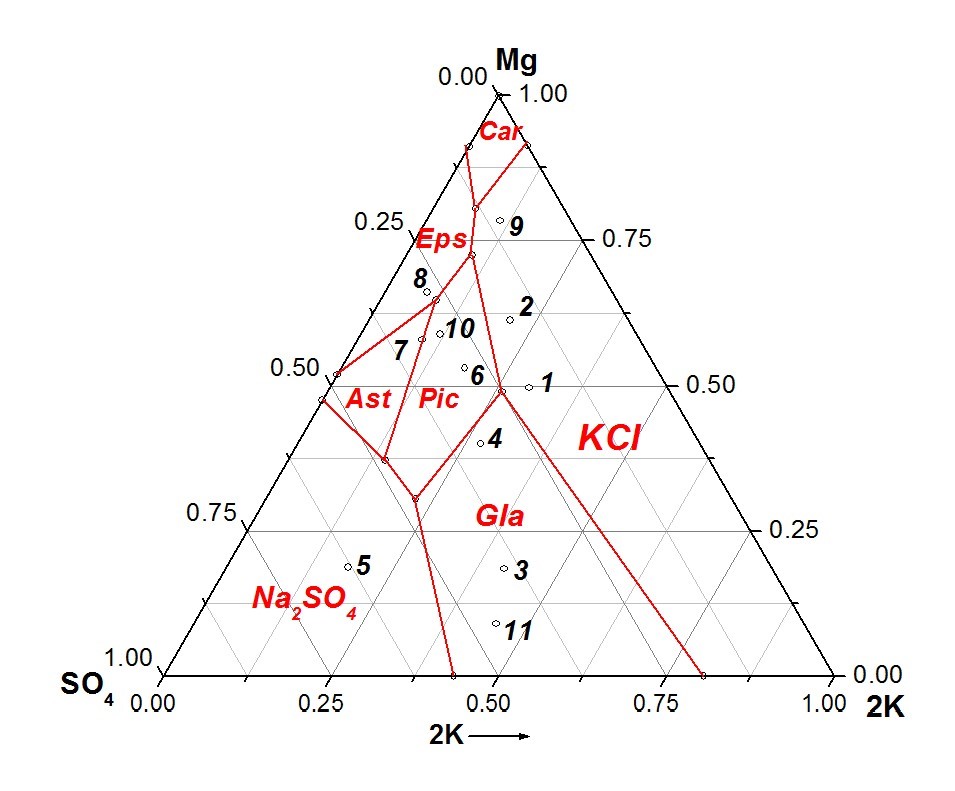
Fig. 1. Position of brine on the metastable phase diagram of Na, K, Mg / Cl, SO42--H2O system at 25 ℃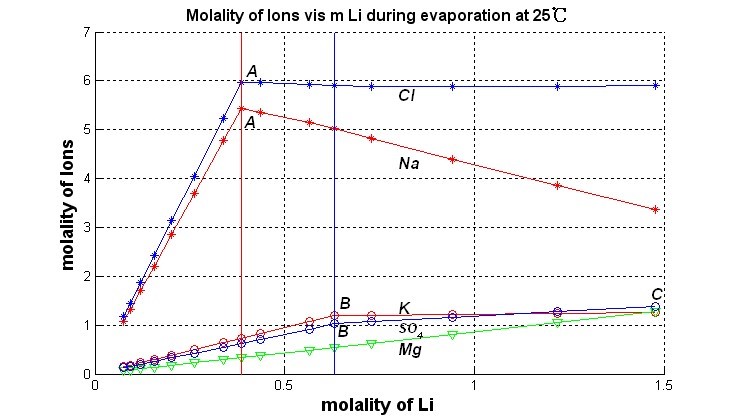
Fig. 2. Variation of brine composition in Lagocuo Salt Lake during constant temperature evaporation at 25 ℃