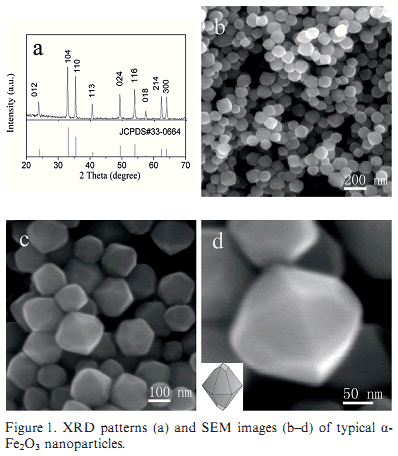
Octadecahedral alpha-Fe2O3 nanoparticles were hydrothermally synthesized in the presence of formamide. These octadecahedral alpha-Fe2O3 nanoparticles have a threefold axis and are enclosed by twelve dominant {112} facets and six {104} facets. In comparison with the {104} facets, the sterically less hindered {112} facets induced the selective adsorption of formamide more easily, which resulted in the gradual increase in the amount of {112} facets by decreasing the amount of {104} facets with increasing formamide. The coercivity (Hc) of these octadecahedral particles increased successively as the aspect ratio (c/a) increased. The sensing capability of these particles toward H2O2 was investigated, and the response currents of the particles dominated by {112} facets showed a linear relationship with a concentration of H2O2 in the range from 40 mu M to 6.66 mM in different stages.