Numerous studies have shown that the hydration and association of borate ions are important for understanding the deposition and crystallization processes of borate minerals, the preservation and flame retardancy of borate solutions for wood, and the performance of boric acid-pressurized nuclear reactors (PWRs). effect. However, there is no clear report on hydration and association of borate ions at the atomic/molecular level.
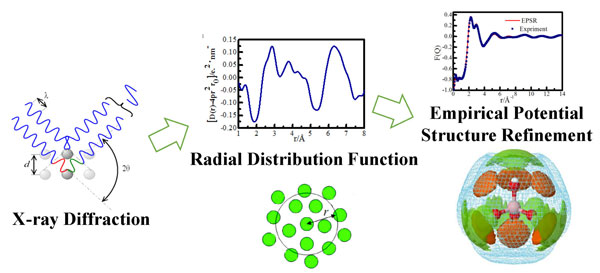
The solution structure team of Salt Lake Resources Chemistry Laboratory of ISL cooperated with the Yamaguchi research group of Fukuoka University in Japan. The hydration and association of borate ions in sodium metaborate solution were investigated by X-ray scattering at a wide range of concentrations. X-ray diffraction and curved imaging plate detectors were used to measure the X-ray scattering intensity of sodium metaborate solution. The 2D scattering pattern was transformed into 1D intensity data and the structural factor was extracted. The empirical potential energy based on Monte Carlo method was used. The structural parameters were refined to reduce the deviations between the experimental and structural factors generated by the structural simulations, and the borate ion hydration and the borate and sodium ion association structures were successfully analyzed. At the same time, in order to obtain structural details, the calculation of the density functional theory (DFT) of the associative cluster of sodium borate was also verified. The research work was published in Phys. Chem. Chem. Phys. (http://pubs.rsc.org/en/content/articlelanding/2017/cp/c7cp05107g.). Recently, the paper was selected by Advance In Engineering as a Key Scientific Article https://advanceseng.com/applied-physics/boh4-hydration-asso.